Abstract
Introduction: Liquid biopsy has been reported to be useful in predicting residual disease in patients with diffuse large B-cell lymphoma (DLBCL). Most of the studies focused on quantifying the level of circulating lymphoma-specific DNA. We explored the clinical relevance of the specific mutated genes in predicting progression in patients with DLBCL.
Method: Peripheral blood samples were collected from patients with DLBCL based on their visit to clinic without other specific selection. Median age of patients is 69 (range 28-91), with 51% of the patients being male. These patients were treated on multiple protocols including R-CHOP, R-EPOCH, Magrath, HCVAD, CAR-T (#2 patients), and others. cfDNA was extracted and sequenced by next generation sequencing using 177 gene panel. The panel uses single primer extension (SPE) approach with UMI. Sequencing depth is increased to more than 2000X after removing duplicates. Low level mutations are confirmed by inspecting BAM file.
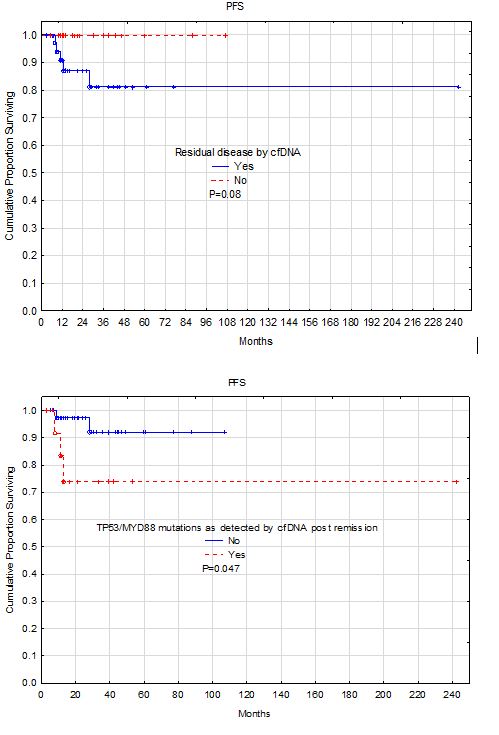
Results: A total of 86 sample from 61 patients were collected post clinical remission at different time points (median 28 weeks, range: 1-994 weeks). Of these samples, 56 (65%) from 46 patients (75%) were positive. However, 6 of these samples from 4 patients had germline mutations or mutations in TET2, ASXL1, or DNMT3A that are consistent with CHIP (clonal hematopoiesis of indeterminate potential). The remaining 50 positive samples from 42 patients had 8 repeats on the same patients collected at different time points. Comparing the 19 negative patients with the 42 positive patients post-remission, patients with residual molecular disease were significantly older than patients without residual disease (P=0.01). However, there was no significant difference between the two groups in gender, ethnic background, LDH, cell of origin classification, or TP53 positivity by IHC. Patients with residual disease showed tendency for short progression-free survival (P=0.08). Focusing on patients with specific mutations detected in the cfDNA showed that 14 (23%) patients had mutations either in TP53 or MYD88. There was no significant difference in age between these two groups or any of the other clinical variables. However, patients with TP53/MYD88 mutations had significantly shorter survival (P=0.04).
Conclusion: Post-remission residual disease as measured by circulating cfDNA is an independent predictor of potential relapse in patients with DLBCL. However, presence of it is important to determine the aggressiveness of the residual circulating clone. Residual circulating lymphoma DNA with TP53 or MYD88 mutations is a strong predictor of earlier relapse.
Pecora: Genetic testing cooperative: Other: equity investor; Genetic testing cooperative: Membership on an entity's Board of Directors or advisory committees. Feldman: Alexion, AstraZeneca Rare Disease: Honoraria, Other: Study investigator. Goy: Bristol Meyers Squibb: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; MorphoSys: Honoraria, Other; AbbVie/Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria; Acerta: Consultancy, Research Funding; Elsevier's Practice Update Oncology, Intellisphere, LLC(Targeted Oncology): Consultancy; Celgene: Consultancy, Honoraria, Research Funding; Michael J Hennessey Associates INC: Consultancy; Elsevier PracticeUpdate: Oncology: Consultancy, Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Medscape: Consultancy; Gilead: Membership on an entity's Board of Directors or advisory committees; Genentech/Hoffman la Roche: Research Funding; AbbVie/Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; OncLive Peer Exchange: Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xcenda: Consultancy, Honoraria; Vincerx pharma: Membership on an entity's Board of Directors or advisory committees; Rosewell Park: Consultancy; LLC(Targeted Oncology): Consultancy; Genomic Testing Cooperative: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership role; Xcenda: Consultancy; Hoffman la Roche: Consultancy; Incyte: Honoraria; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Infinity/Verastem: Research Funding; Janssen: Research Funding; Karyopharm: Research Funding; Vincerx: Honoraria, Membership on an entity's Board of Directors or advisory committees; Physicians' Education Resource: Consultancy, Other: Meeting/travel support; COTA (Cancer Outcome Tracking Analysis): Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership role; Phamacyclics: Research Funding; Constellation: Research Funding; Hackensack Meridian Health, Regional Cancer Care Associates/OMI: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal